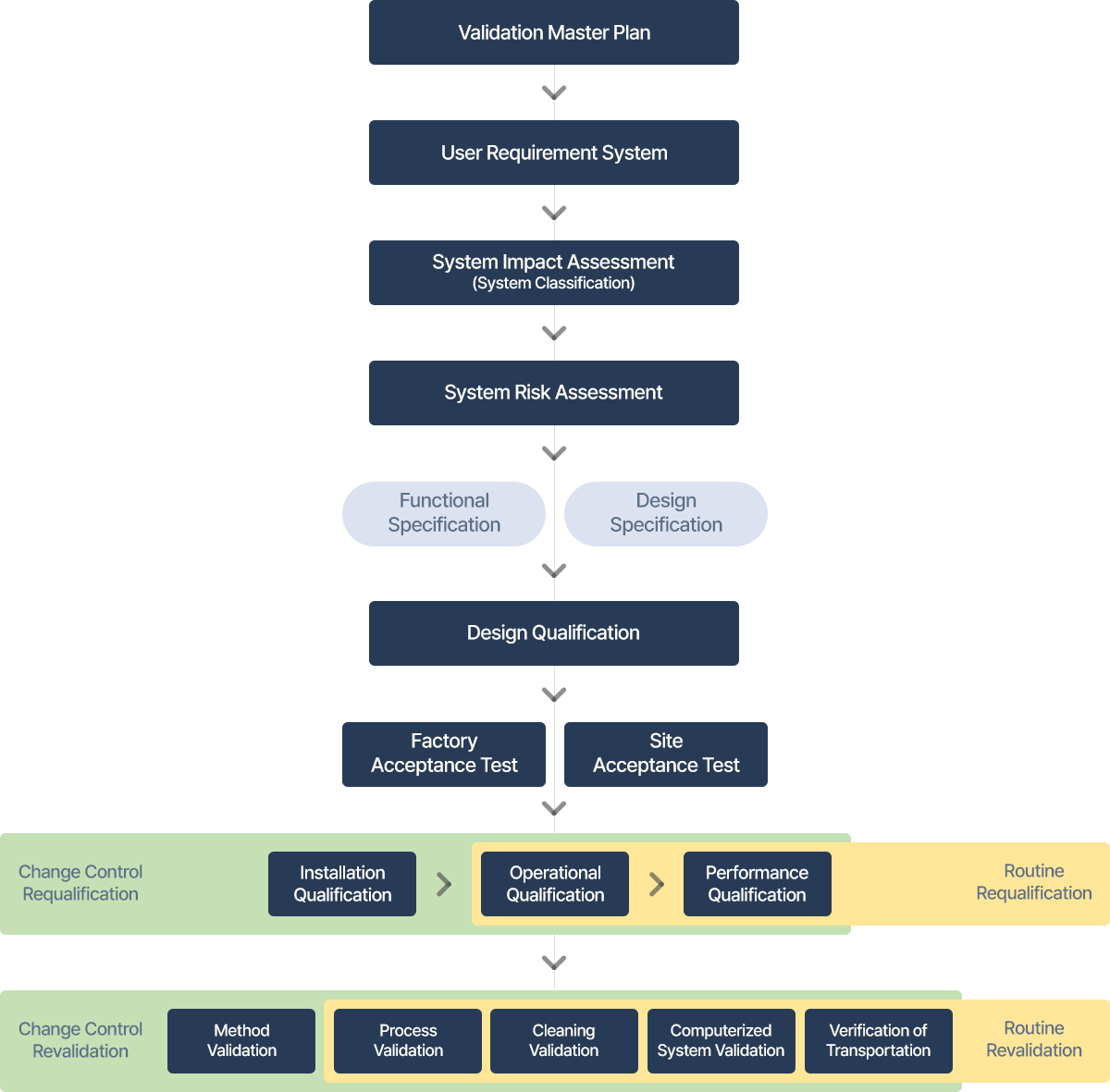
proves the planned design of the facility, system and equipment is suitable for the purpose of usage.
proves that the installation or reconfigured facility, system and equipment is in accordance with the recommendations of the manufacturer and/or the approved design.
proves that the installation or reconfigured facility, system and equipment is operating within the anticipated operating range as intended
proves that the system, facility can operate effectively and similarly based on the approved process methods and product specification.
is an adequate routine assessment to confirm that the equipment, facility, utilities and system are maintained in accordance with the required status.
·Evaluate the equipment’s potential risk and impact by deriving
the CQA (Critical Quality Attribute) and CPP (Critical Process Parameter)
·When conducting RQ, set the scope and items of Execution
through Risk Assessment.
·Write a protocol by selecting the items of Execution according
to the scope and phase that is derived from the Risk
Assessment
·Execute in accordance with the protocol prepared/reviewed/
approved according to risk analysis
·Qualified personnel record derived data after execution on
the report.
·Suitability is determined after comparing the recorded data
and pre-set acceptance criteria.
proves the planned design of the facility, system and equipment is suitable for the purpose of usage.
proves that the installation or reconfigured facility, system and equipment is in accordance with the recommendations of the manufacturer and/or the approved design.
proves that the installation or reconfigured facility, system and equipment is operating within the anticipated operating range as intended
proves that the system, facility can operate effectively and similarly based on the approved process methods and product specification.
is an adequate routine assessment to confirm that the equipment, facility, utilities and system are maintained in accordance with the required status.
·Execute Process Risk Assessment in accordance with the
guidelines of ICH Q9(Quality Risk Management)
·Reflect the Risk Assessment results when
writing Validation Protocol (Process, Cleaning,
Testing and Analysis, Computerized System,
Transportation)
·Clients will conduct manufacturing, cleaning, measuring
and analyzing
·Provide support services when deviation occurs
·Draft the report based on the result of
the execution