is a concept for all that may serve as a yardstick by regularizing and documenting the relevant records and procedures so that the standard for product quality may be established and thereby maintained.
sureAssist provides QMS consulting services on establishing document system through RA(Risk Assessment) and applying DI (Data Integrity).
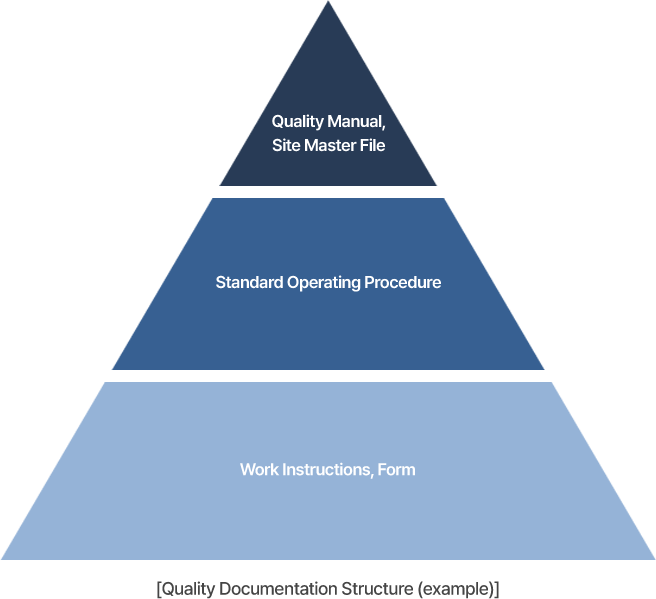
It includes writing/revising documents such as Quality Manual, SOP, and Procedure, etc.

Identify the status of document system
Identify client’s demand on the system to be established
Identify relevant regulation
Establishing a document structure, document tree, and list of revisions based on identified requirements and regulations.
Draft a document that reflects RA(Risk Assessment) and DI(Data Integrity)
Conduct a training for SOP operation that obtained final approval by the client
sureAssistmaintains readiness posture to analyze new regulations on GMP & GxP and supports our clients to be prepared for the changes.

EU GMP Annex 1 and Aseptic Pharmaceutical Manufacturing
(Annex 1) - Gap Analysis and Application
CCS(Contamination Control Strategy) - Establish the monitoring system and documentation by developing the CCS that reflects the guidelines
DI(Data Integrity) Gap Analysis - Identify the present condition on how the client’s current data of equipment system and document archive is maintained - Evaluation of the current system based on evaluation items of the Food and Drug Administration, PIC/S, and the Drug Manufacturer's Data Integrity (DI) evaluation guidelines issued abroad - Review the equipment operating system and the document system in accordance with the principles of ALCOA+